Pew Research Center conducted this study to understand how Americans are continuing to respond to the coronavirus outbreak. For this analysis, we surveyed 10,093 U.S. adults from Sept. 8 to 13, 2020. This report also draws on data from a survey fielded April 29 to May 5, 2020, among 10,957 U.S. adults.
Everyone who took part in either survey is a member of Pew Research Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology.
See here to read more about the questions used for this report, along with responses, and its methodology.
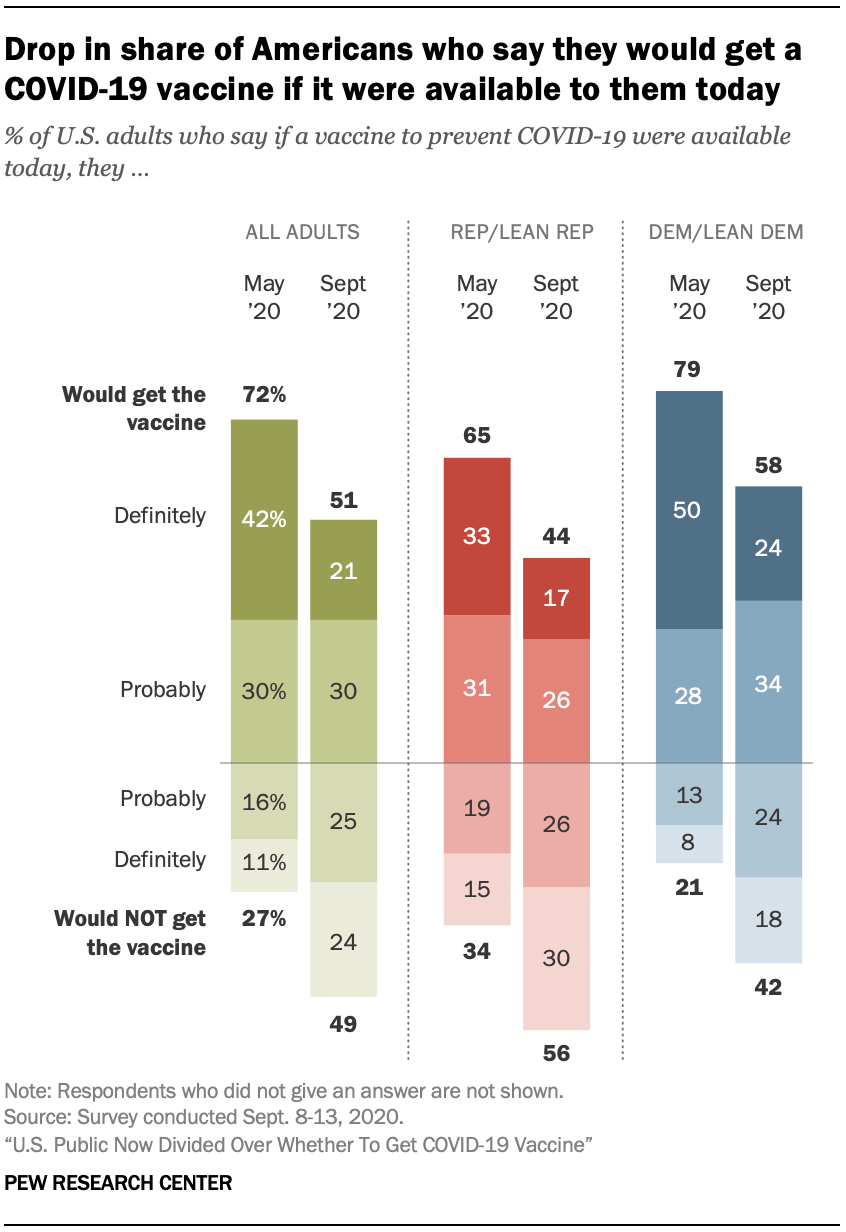
As efforts to develop and test a COVID-19 vaccine spur debate around the timing and release of a federally approved vaccine, the share of Americans who say they would get vaccinated for the coronavirus has declined sharply since earlier this year.
About half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today; nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May, a 21 percentage point drop.
The share who would definitely get a coronavirus vaccine now stands at just 21% – half the share that said this four months ago.
There are widespread public concerns about aspects of the vaccine development process. On the heels of a pledge from nine pharmaceutical companies to ensure that a potential vaccine would meet rigorous standards, the Center survey finds three-quarters of Americans (77%) think it’s very or somewhat likely a COVID-19 vaccine will be approved in the United States before its safety and effectiveness are fully understood. And when asked about the pace of the vaccine approval process, 78% say their greater concern is that it will move too fast, without fully establishing safety and effectiveness, compared with just 20% who are more concerned approval will move too slowly, creating unnecessary delays.
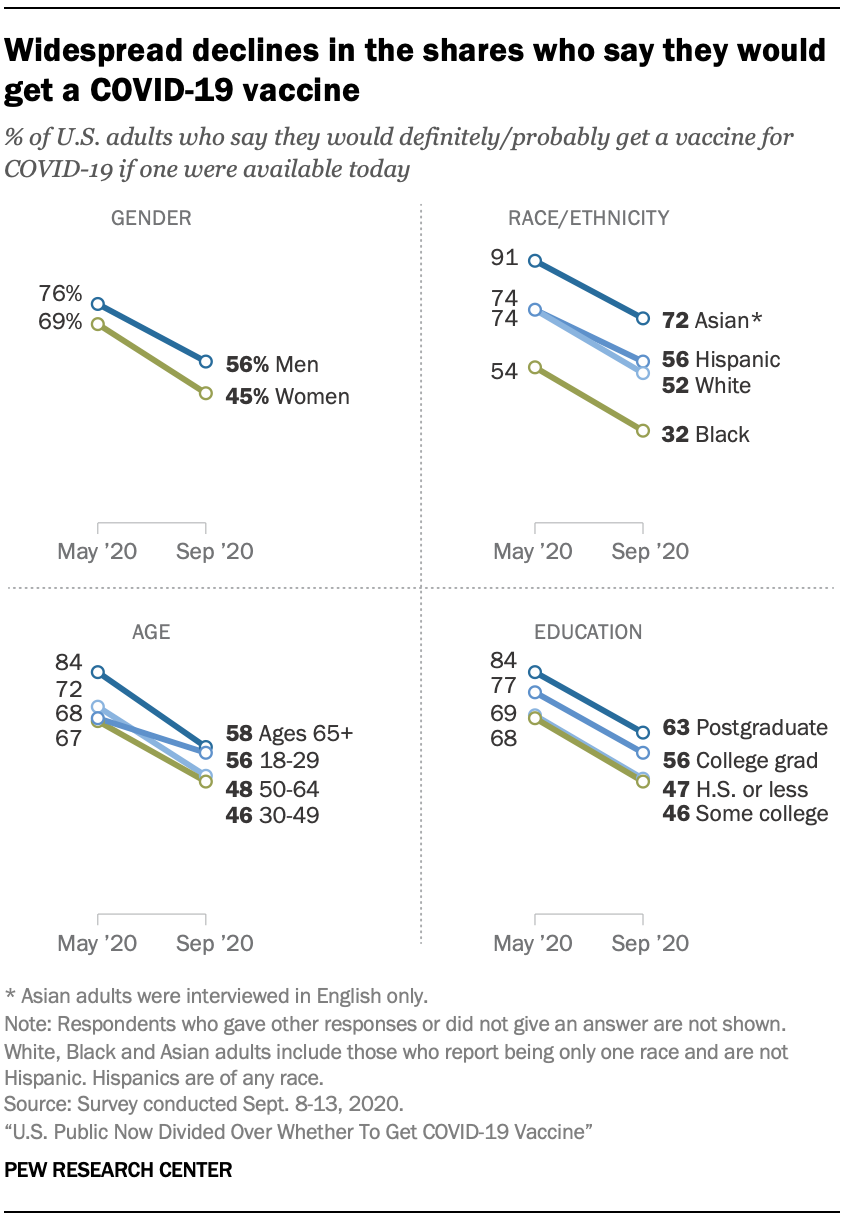
The new national survey by Pew Research Center, conducted Sept. 8-13 among 10,093 U.S. adults, finds intent to get a COVID-19 vaccine has declined across all major political and demographic groups.
However, sizable differences across groups remain. Democrats and those who lean to the Democratic Party are 14 percentage points more likely than Republicans and Republican leaners to say they would probably or definitely get a vaccine (58% vs. 44%). And Black adults are much less likely to say they would get a vaccine than other Americans: Just 32% of Black adults say they would definitely or probably get a COVID-19 vaccine, compared with 52% of White adults, 56% of Hispanics and nearly three-quarters (72%) of Asian Americans. (Asian adults were interviewed in English only.)
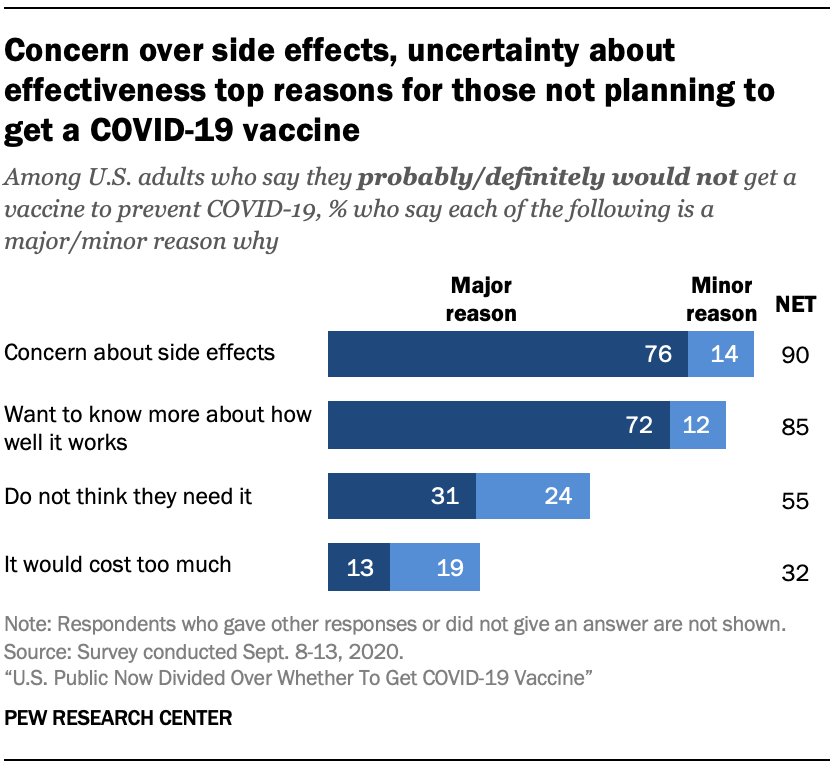
Concerns about side effects and uncertainty around the effectiveness of a vaccine are widely cited as reasons by those who would not get a COVID-19 vaccine if one were available today.
Among the roughly half of Americans who say they would not get a COVID-19 vaccine, 76% say concern about side effects is a major reason why they would definitely or probably not get it.
Several vaccines are currently under trial right now. One trial was temporarily put on hold earlier this month for potentially causing side effects in a trial participant, but has since resumed.
A large majority (72%) of those who would not get a COVID-19 vaccine also say a desire to know more about how well it would work is a major reason why they don’t currently plan to get a coronavirus vaccine.
Fewer adults cite not thinking they need the vaccine (31%) or the vaccine’s cost (13%) as a major reason they would not likely get vaccinated.
Those who say they would definitely or probably get a vaccine for COVID-19 if it were available today see a range of factors that could impact that decision.
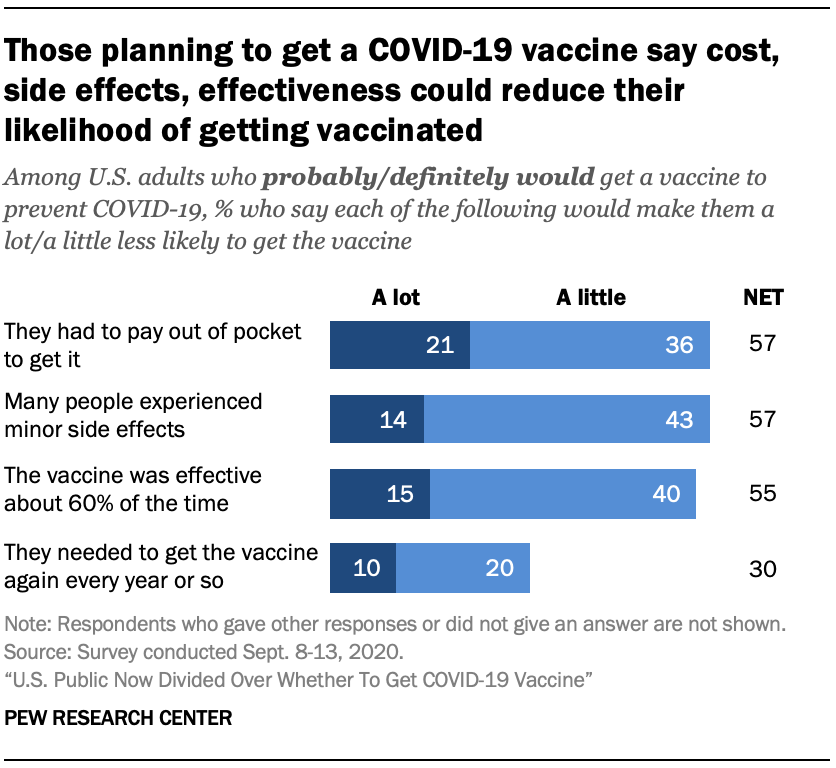
Overall, 57% of those planning to get a vaccine say they would be a little (36%) or a lot (21%) less likely to do so if they had to pay out of pocket to get it. About four-in-ten (42%) say out-of-pocket costs would not change their likelihood of getting a vaccine.
Similarly, majorities say that many people experiencing minor side effects (57%) and the vaccine being effective about 60% of the time (55%) would reduce the likelihood of them getting vaccinated at least a little. But fewer than two-in-ten say either of these things would make them a lot less likely to get the vaccine. The possible need to get a vaccine again every year or so is not seen as a major deterrent among those planning to get vaccinated: 70% say this wouldn’t make a difference to them.
Researchers are still not sure how effective a COVID-19 vaccine will ultimately be. The U.S. Food and Drug Administration has said it would authorize a COVID-19 vaccine if it was safe and at least 50% effective in preventing the disease or decreasing the severity of infections, although Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, has said scientists are hoping for a vaccine that is at least 75% effective.
Many Americans think it’s likely a vaccine will be used before its safety and effectiveness are fully understood
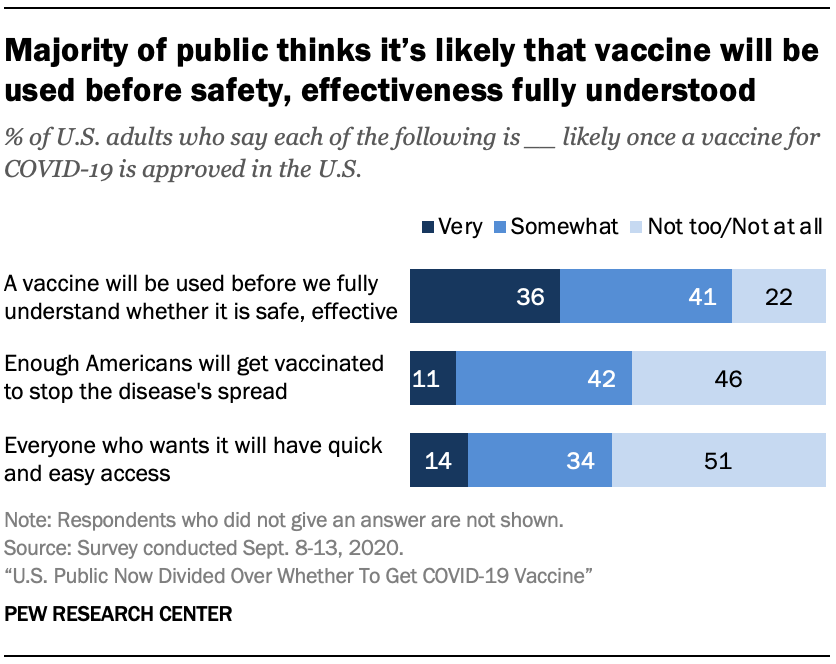
As Americans look ahead to when a vaccine for COVID-19 is approved in the U.S., many express doubts about how safe and effective a vaccine will be initially.
About three-quarters of Americans (77%) say it is at least somewhat likely that a vaccine for COVID-19 will be approved and used in the U.S. before it’s fully known whether it is safe and effective, including 36% who say this is very likely to happen. Just 22% say this is not too or not at all likely.
Public assessments are more mixed when it comes to whether enough Americans will get vaccinated to curb the spread of the disease: 53% say this is at least somewhat likely, while 46% think it not too or not at all likely.
Americans also have a mixed outlook on vaccine access. About half of U.S. adults (48%) say it’s at least somewhat likely that everyone who wants the vaccine will have quick and easy access to it, while 51% say this is not too or not at all likely.
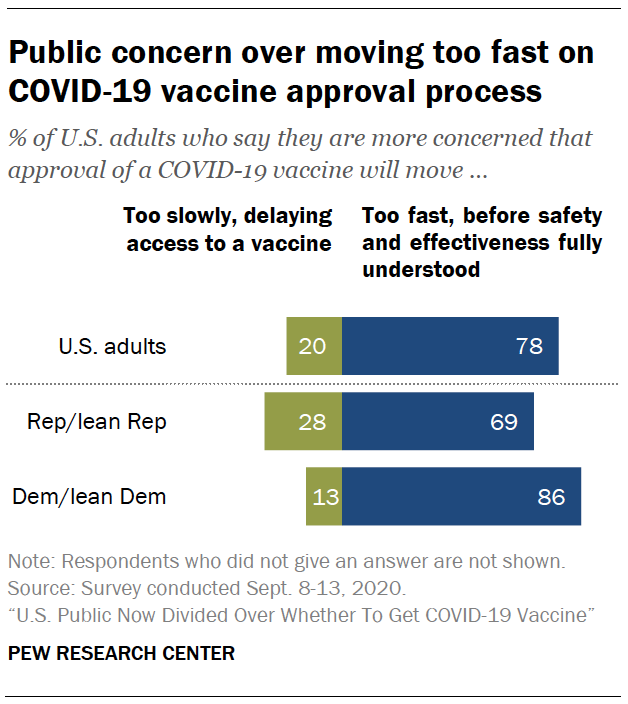
Consistent with the view that a vaccine may be approved before its safety and effectiveness are fully understood, Americans overwhelmingly say their greater concern is that the approval process will move too fast, rather than too slowly. Nearly eight-in-ten (78%) say their greater concern is that the vaccine approval process will move too fast, without fully establishing that it is safe and effective. Just 20% say they are more concerned the approval process will move too slowly, causing unnecessary delays in access to a vaccine.
While Republicans and Democrats have differed over many aspects of the coronavirus outbreak – including the threat it presents to public health and how quickly to lift restrictions on public activity – majorities of both groups say their greater concern about the vaccine approval process is that it will move too fast, rather than too slowly. About seven-in-ten Republicans (69%) are more concerned about the approval process moving too fast, and an even larger majority of Democrats (86%) share this view.
Intent to get COVID-19 vaccine tied to confidence in development process
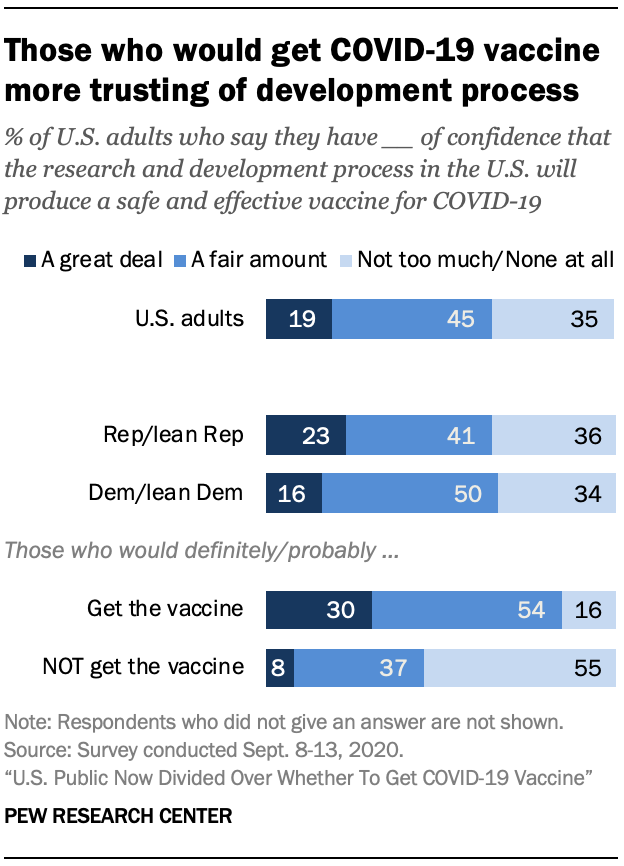
Those who plan to get a COVID-19 vaccine express much greater confidence in the vaccine development process than those who do not plan to get vaccinated.
Overall, 19% of the public has a great deal of confidence that the research and development process in the U.S. will produce a safe and effective vaccine for COVID-19, while another 45% say they have a fair amount of confidence. About a third (35%) say they have not too much or no confidence in this process.
Among those who say they would definitely or probably get a vaccine, more than eight-in-ten express either a great deal (30%) or a fair amount (54%) of confidence in the research and development process. By contrast, 55% of those not planning to get a coronavirus vaccine say they have not too much or no confidence at all in this process.




